TECENTRIQ
DOSING &
ADMINISTRATION
Non-small cell lung cancer (NSCLC)
Indications1
TECENTRIQ, in combination with bevacizumab, paclitaxel and carboplatin, is indicated for the first-line treatment of adult patients with metastatic non-squamous non-small cell lung cancer (NSCLC). In patients with EGFR mutant or ALK-positive NSCLC, TECENTRIQ, in combination with bevacizumab, paclitaxel and carboplatin, is indicated only after failure of appropriate targeted therapies.
TECENTRIQ, in combination with nab-paclitaxel and carboplatin, is indicated for the first-line treatment of adult patients with metastatic non-squamous NSCLC who do not have EGFR mutant or ALK-positive NSCLC.
TECENTRIQ as monotherapy is indicated for the treatment of adult patients with locally advanced or metastatic NSCLC after prior chemotherapy. Patients with EGFR mutant or ALK+ NSCLC should also have received targeted therapy before receiving TECENTRIQ.
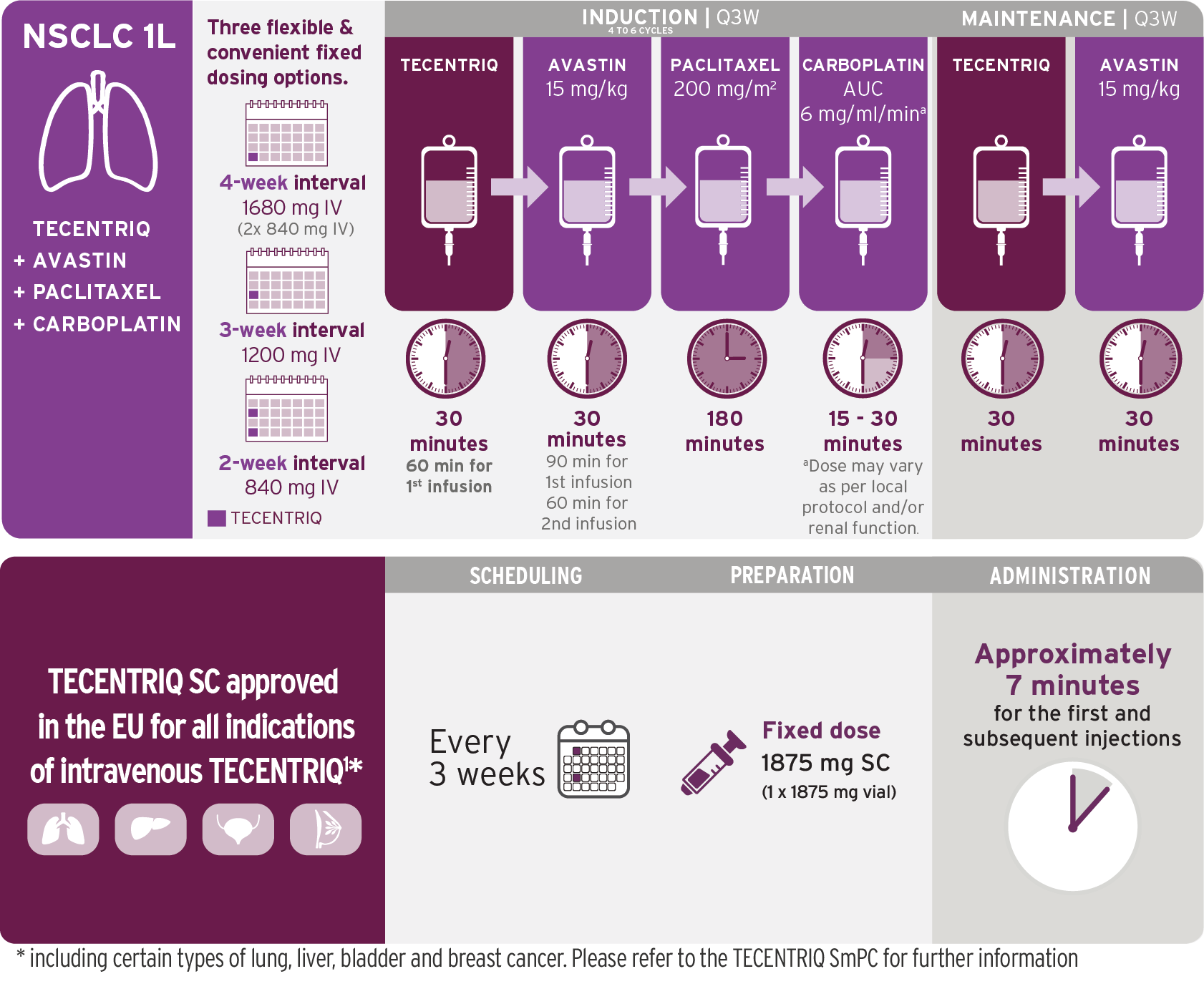
1L Metastatic non-squamous NSCLC
TECENTRIQ in combination with bevacizumab, paclitaxel and carboplatin.
A consistent infusion schedule with chemotherapy-free post-induction dosing.
Infusions once every 3 weeks1,2
1L=first line; AUC=area under the concentration-time curve; IV=intravenous; NSCLC=non-small cell lung cancer.
Based on the dosing schedule from IMpower150.2 Visualization of vials is illustrative and does not represent actual vial usage.
*In patients of Asian race/ethnicity, the paclitaxel dose was lowered from 200 mg/m2 to 175 mg/m2.
- TECENTRIQ should be administered prior to bevacizumab, paclitaxel, and carboplatin on Day 1 of each cycle
- Administer the initial infusion of TECENTRIQ over 60 minutes; if the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes
- Do not administer TECENTRIQ as an IV push or bolus
- Do not co-administer other drugs through the same IV line
- Refer to the respective prescribing information for bevacizumab, paclitaxel, and carboplatin for recommended dosing information
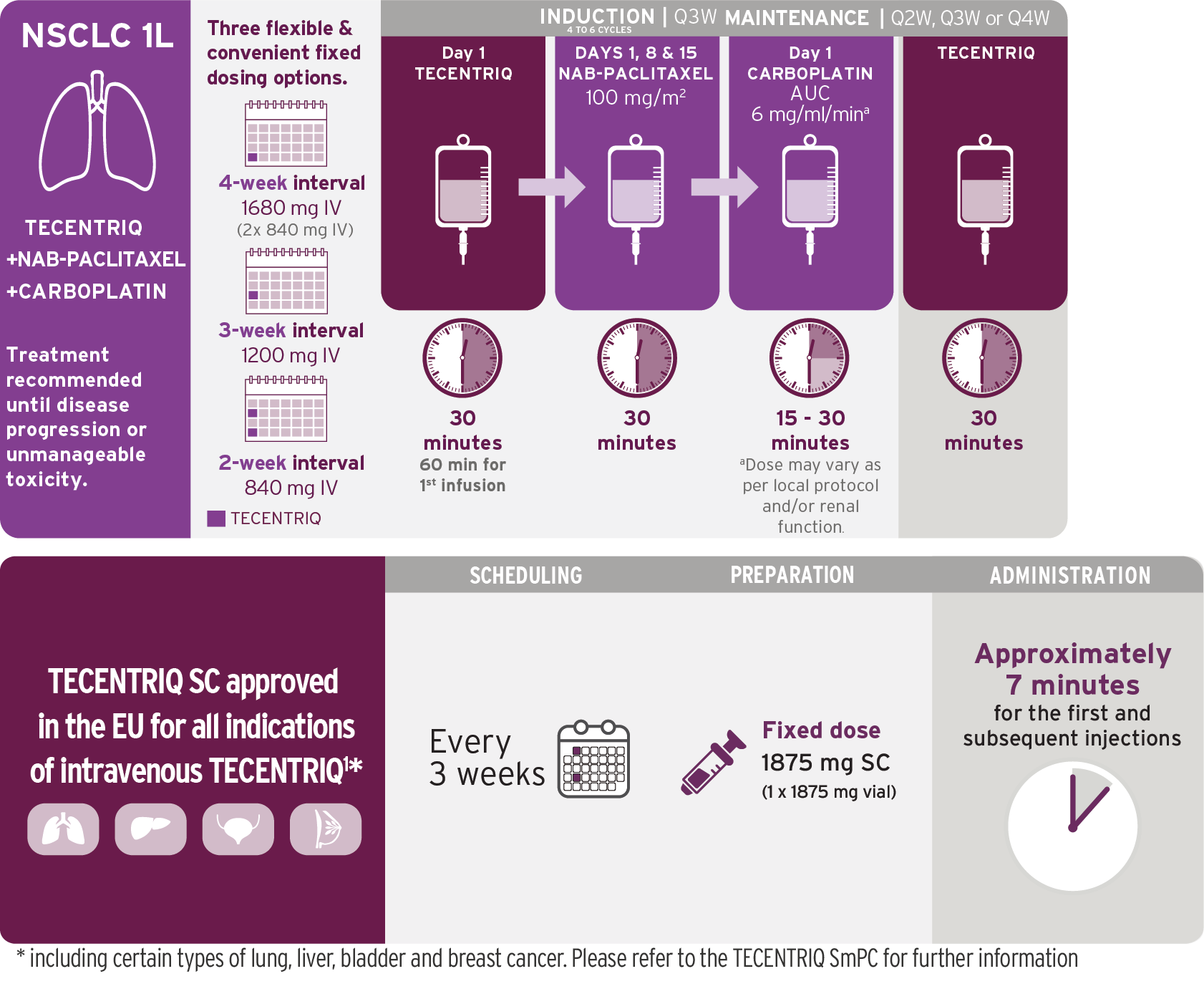
TECENTRIQ in combination with nab-paclitaxel and carboplatin.
Induction phase followed by chemotherapy-free infusions with TECENTRIQ.
Infusions once every 3 weeks1,3
1L=first line; AUC=area under the concentration-time curve; IV=intravenous; nab-pac=nanoparticle albumin–bound paclitaxel; NSCLC=non-small cell lung cancer.
Based on the dosing schedule from IMpower130.3 Visualization of vials is illustrative and does not represent actual vial usage.
- For each 21-day cycle, TECENTRIQ, nab-pac, and carboplatin are administered on Day 1; in addition, nab-pac is administered on days 8 and 15
- Administer the initial infusion of TECENTRIQ over 60 minutes; if the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes
- Do not administer TECENTRIQ as an IV push or bolus
- Do not co-administer other drugs through the same IV line
- Refer to the respective prescribing information for the combination products
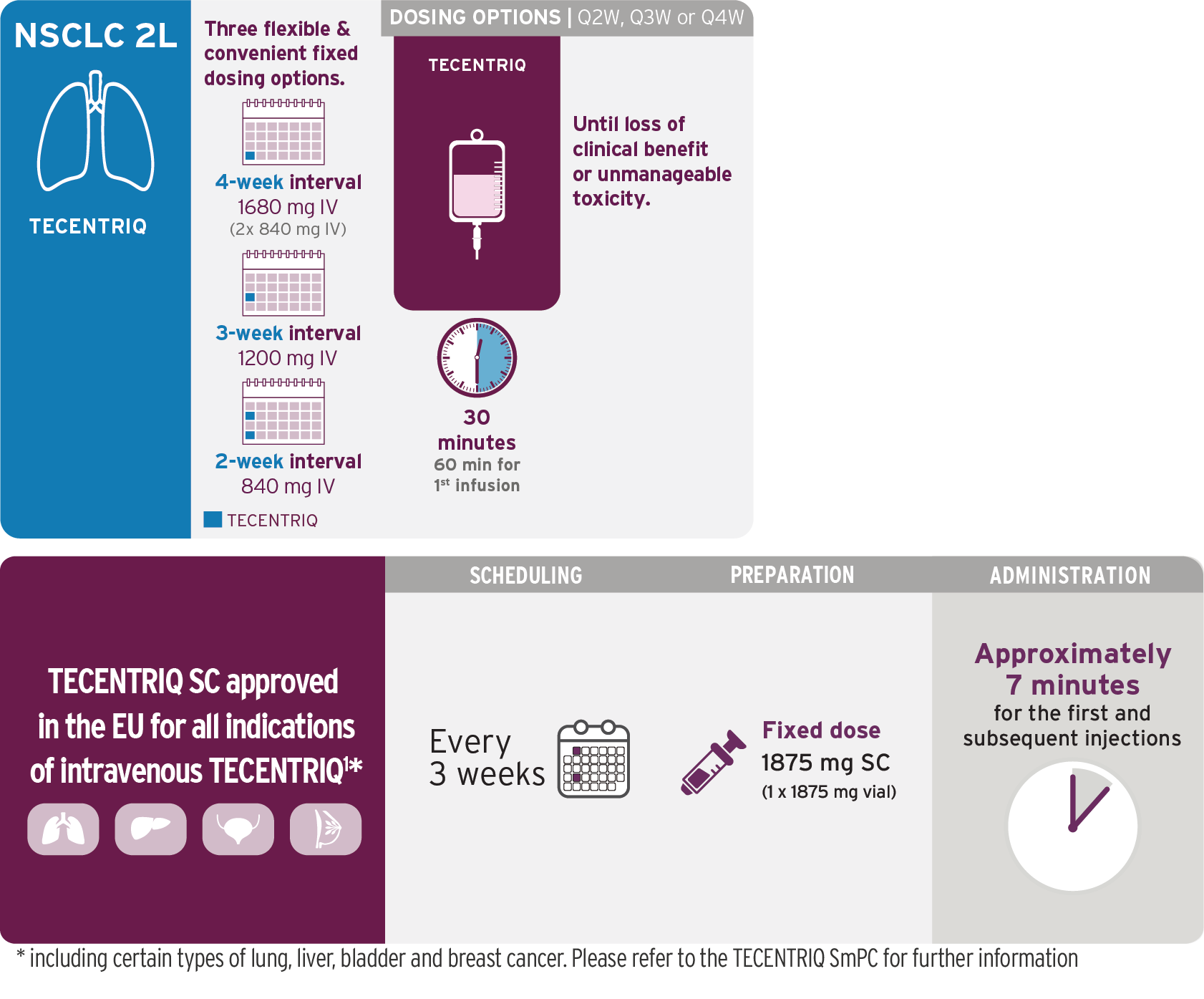
2L Metastatic NSCLC
TECENTRIQ as monotherapy
TECENTRIQ offers flexible dosing options
Choose the most suitable infusion schedule for your patients1,4
Q4W dosage is administered with two 840-mg vials of TECENTRIQ.
Visualization of vials is illustrative and does not represent actual vial usage.
2L=second line; IV=intravenous; Q4W=every 4 weeks.
- Do not administer TECENTRIQ as an IV push or bolus
- Do not co-administer other drugs through the same IV line
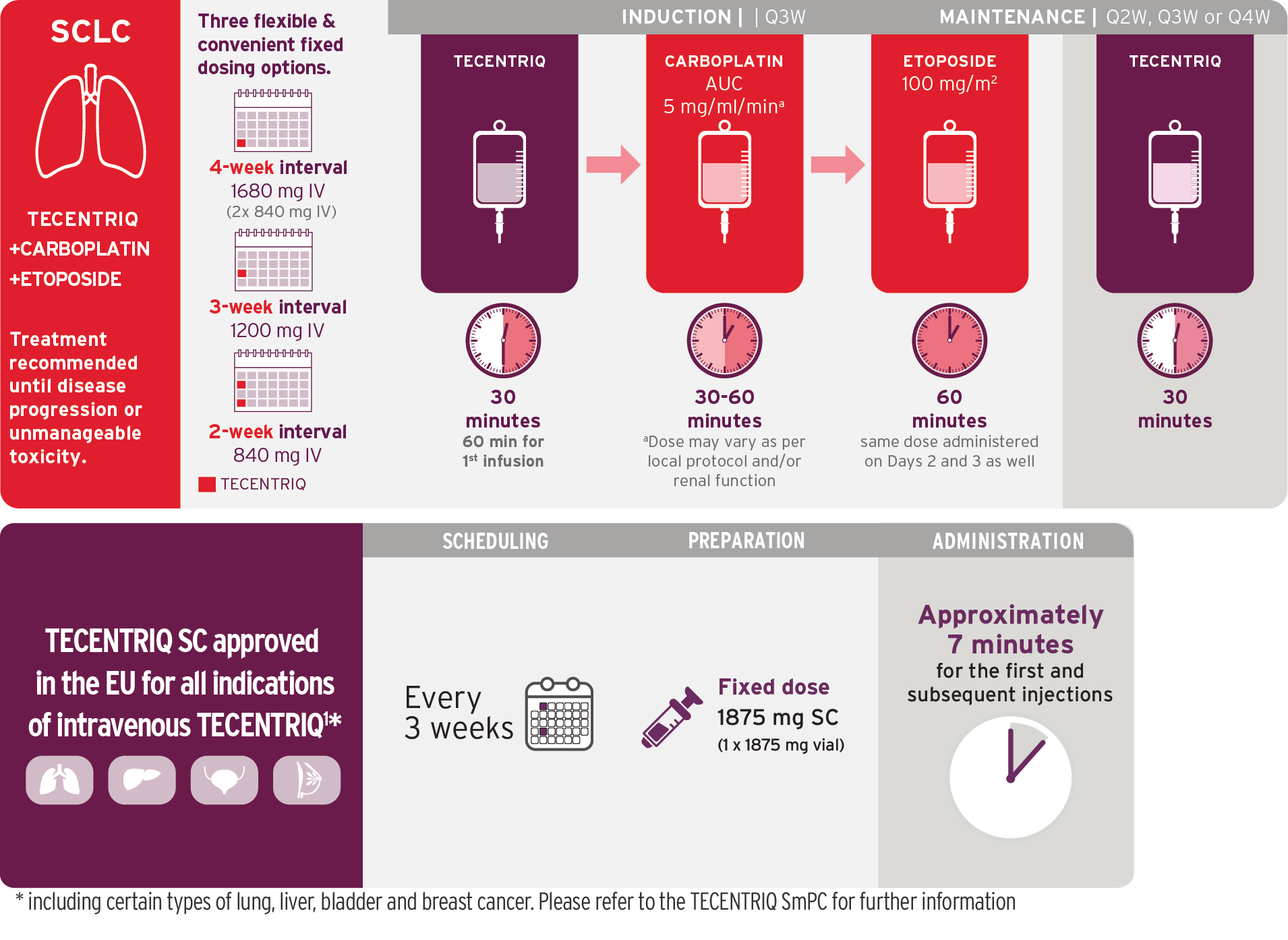
Small Cell Lung Cancer (SCLC)
Indications1
TECENTRIQ, in combination with carboplatin and etoposide, is indicated for first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
TECENTRIQ, in combination with carboplatin and etoposide
TECENTRIQ Infusion Schedule with Chemotherapy-Free Maintenance
Q3W combination induction followed by flexible dosing options in maintenance1,5
Dosing information for carboplatin/etoposide is based on IMpower133 trial;
TECENTRIQ was administered Q3W in IMpower133.5 Visualization of vials is illustrative and does not represent actual vial usage.
Q3W=every 3 weeks
- During induction phase, TECENTRIQ should be administered by IV infusion first, followed by carboplatin, then etoposide
- During maintenance phase, TECENTRIQ can be administered as 1200 mg every 3 weeks
- Administer the initial infusion of TECENTRIQ over 60 minutes; if the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes
- Do not administer TECENTRIQ as an IV push or bolus
- Do not co-administer other drugs through the same IV line
- Refer to the respective prescribing information for carboplatin and etoposide for recommended dosing information
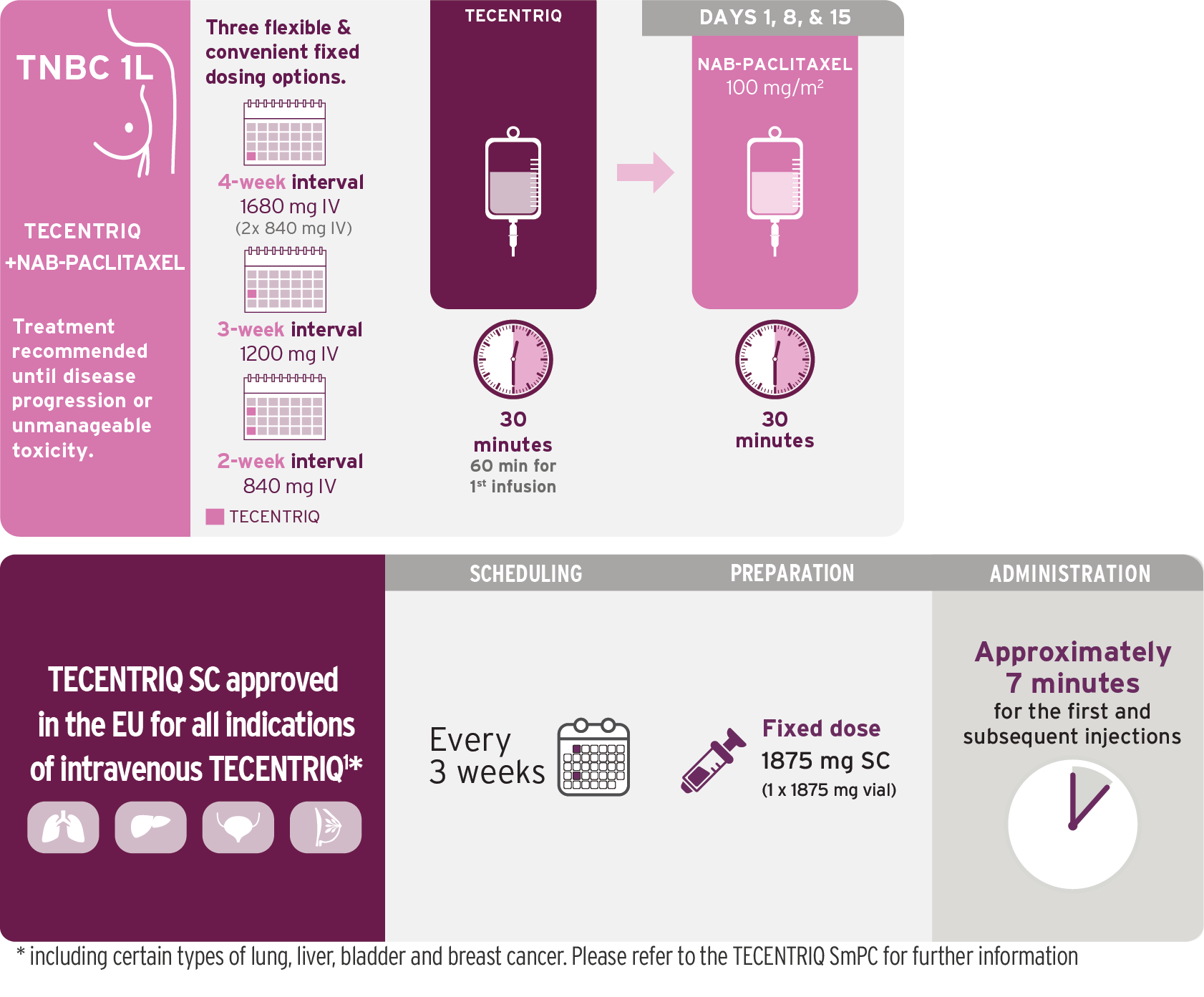
Triple-negative Breast Cancer (TNBC)
Indication4
TECENTRIQ in combination with nab-paclitaxel (nab-pac) is indicated for the treatment of adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) whose tumours have PD-L1 expression ≥1% and who have not received prior chemotherapy for metastatic disease.
TECENTRIQ in combination with nab-paclitaxel.
Dosing Schedule Allows Patients an Infusion-Free Week Between Cycles
28-day dosing cycles continue until disease progression or unacceptable toxicity4,6
nab-pac=nanoparticle albumin–bound paclitaxel.
Based on the dosing schedule from IMpassion130.6
Visualization of vials is illustrative and does not represent actual vial usage.
- For each 28-day cycle, TECENTRIQ is administered on Days 1 and 15, and nab-pac 100 mg/m2 is administered on Days 1, 8, and 15
- On Days 1 and 15, TECENTRIQ should be administered first, followed by nab-pac
- Administer the initial infusion of TECENTRIQ over 60 minutes; if the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes
- Do not administer TECENTRIQ as an IV push or bolus
- Do not co-administer other drugs through the same IV line
- TECENTRIQ and nab-pac may be discontinued for toxicity independently of each other
- See also the prescribing information for nab-pac prior to initiation
840-mg single-dose vial now approved for TECENTRIQ
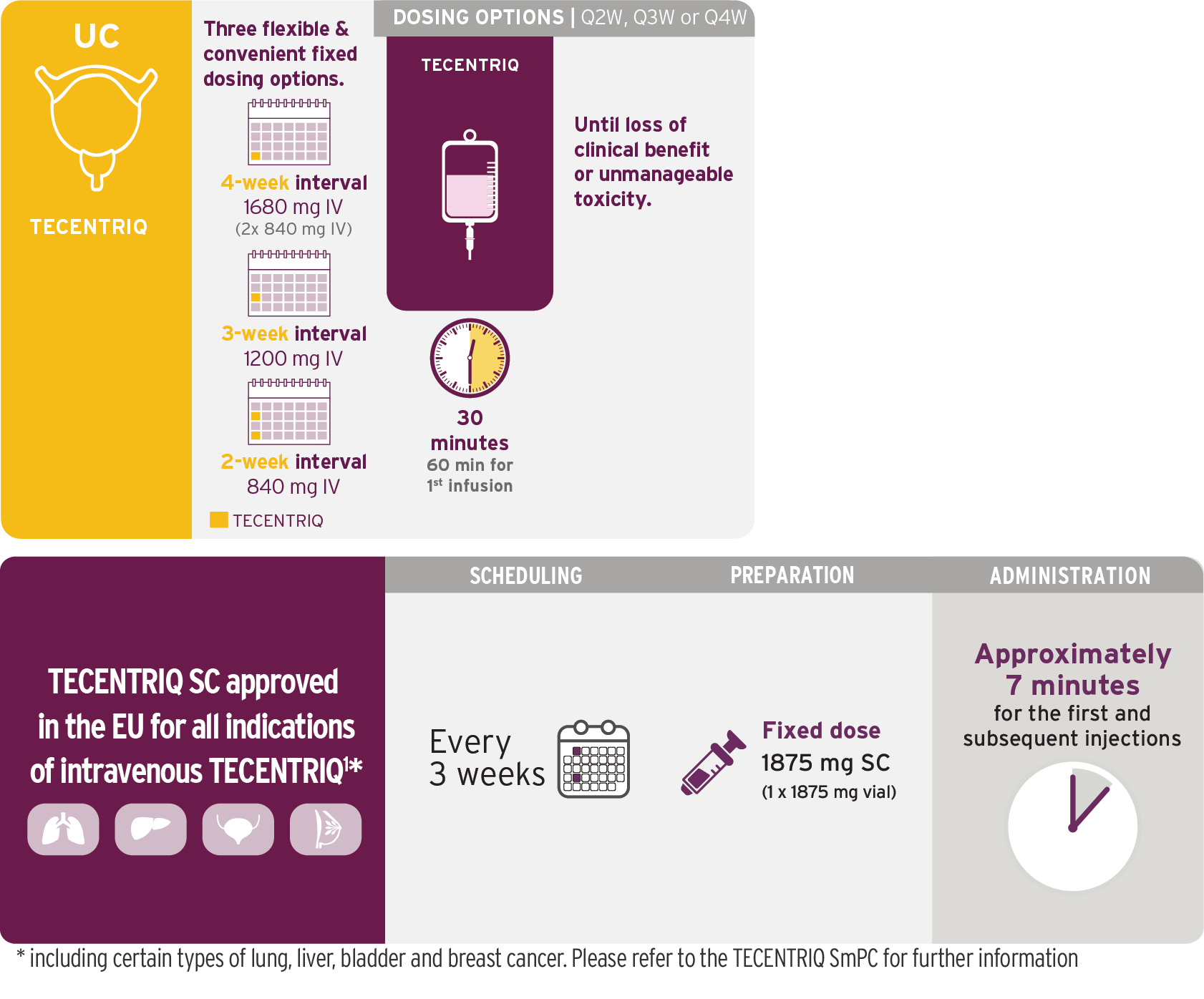
Urothelial carcinoma
Indications1,4
TECENTRIQ as monotherapy is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma (mUC):
- After prior platinum containing chemotherapy, or
- Who are considered cisplatin ineligible, and whose tumours have a PD-L1 expression ≥5%
TECENTRIQ as monotherapy
TECENTRIQ Offers Flexible Dosing Options
Choose the most suitable infusion schedule for your patients1,4
Based on the dosing schedule from IMvigor2107 and IMvigor20118. Visualization of vials is illustrative and does not represent actual vial usage.
Q4W dosage is administered with two 840-mg vials of TECENTRIQ.
IV=intravenous; q3w=every 3 weeks.
- Do not administer as an IV push or bolus
- Do not co-administer other drugs through the same IV line
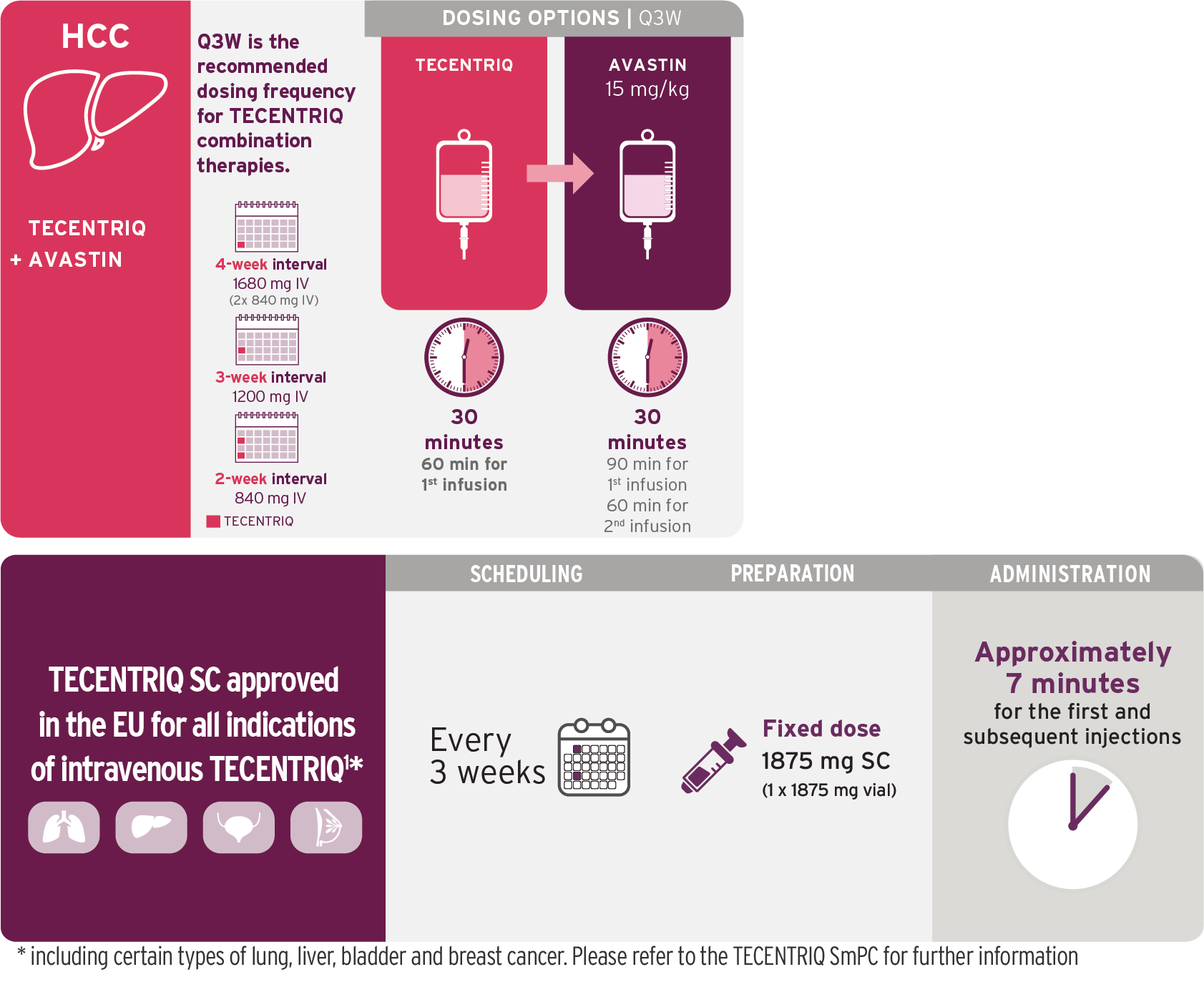
Hepatocellular Carcinoma
A Cancer Immunotherapy combination schedule once every 3 weeks.1
Based on the dosing schedule from IMbrave1509. Visualization of vials is illustrative and does not represent actual vial usage.
- TECENTRIQ should be administered first, followed by bevacizumab
- Administer the initial infusion of TECENTRIQ over 60 minutes; if the first infusion is tolerated, all subsequent infusions may be delivered over 30 minutes
- The recommended dose of TECENTRIQ is 1,200 mg followed by bevacizumab 15 mg/kg of body weight, administered by intravenous infusion every three weeks
- Do not administer TECENTRIQ as an IV push or bolus
- Do not co-administer other drugs through the same IV line
- Refer to the bevacizumab SmPC for prescribing information, dosage modifications or specific adverse reactions
DOSAGE MODIFICATIONS FROM THE TECENTRIQ
PRESCRIBING INFORMATION1†
ADVERSE REACTION |
SEVERITY OF ADVERSE REACTION‡ |
DOSAGE MODIFICATIONS |
|---|---|---|
Pneumonitis |
Grade 2 |
Withhold dose until grade 1 or resolved and corticosteroid dose is less than or equal to prednisone 10 mg per day (or equivalent) |
Grade 3 or 4 |
Permanently discontinue | |
Hepatitis in patients without hepatocellular carcinoma (HCC) |
Grade 2: (ALT or AST > 3 to 5 x upper limit of normal [ULN] or blood bilirubin > 1.5 to 3 x ULN) |
Withhold Tecentriq
Treatment may be resumed when the event improves to Grade 0 or Grade 1 within 12 weeks and corticosteroids have been reduced |
Grade 3 or 4: (ALT or AST > 5 x ULN or blood bilirubin > 3 x ULN) |
Permanently discontinue TECENTRIQ | |
Hepatitis in patients with HCC |
If AST/ALT is within normal limits at baseline and increases to >3x to ≤10x ULN or
If AST/ALT is >1 to ≤ 3x ULN at baseline and increases to >5x to ≤ 0x ULN or
If AST/ALT is >3x to ≤ 5x ULN at baseline and increases to >8x to ≤ 10x ULN |
Withhold Tecentriq
Treatment may be resumed when the event improves to Grade 0 or Grade 1 within 12 weeks and corticosteroids have been reduced |
If AST/ALT increases to >10x ULN or total bilirubin increases to >3x ULN |
Permanently discontinue Tecentriq |
|
| Colitis or diarrhoea |
Grade 2 or 3 |
Withhold dose until grade 1 or resolved and corticosteroid dose is less than or equal to prednisone 10 mg per day (or equivalent) |
| Grade 4 |
Permanently discontinue |
|
| Endocrinopathies§ |
Grade 2, 3, or 4 |
Withhold dose until grade 1 or resolved and clinically stable on hormone replacement therapy |
Other immune-mediated adverse reactions involving a major organ |
Grade 3 |
Withhold dose until grade 1 or resolved and corticosteroid dose is less than or equal to prednisone 10 mg per day (or equivalent) |
Grade 4 |
Permanently discontinue | |
Infections |
Grade 3 or 4 | Withhold dose until grade 1 or resolved |
Infusion-related reactions |
Grade 1 or 2 |
Interrupt or slow the rate of infusion |
Grade 3 or 4 |
Permanently discontinue | |
Persistent grade 2 or 3 adverse reaction (excluding endocrinopathies) |
Grade 2 or 3 adverse reaction that does not recover to grade 0 or 1 within 12 weeks after last TECENTRIQ dose |
Permanently discontinue |
Inability to taper corticosteroid |
Inability to reduce to less than or equal to prednisone 10 mg per day (or equivalent) within 12 weeks after last TECENTRIQ dose |
Permanently discontinue |
Recurrent grade 3 or 4 adverse reaction |
Recurrent grade 3 or 4 (severe or life-threatening) adverse reaction |
Permanently discontinue |
No filter results
ALT=alanine aminotransferase; AR=adverse reaction; AST=aspartate aminotransferase; IV=intravenous; NCI CTCAE=National Cancer Institute Common Terminology Criteria for Adverse Events; ULN=upper limit of normal.
†Consult the nab-pac Prescribing Information for dosage modifications and AR management.
‡NCI CTCAE v4.0.
§Including, but not limited to, hypophysitis, adrenal insufficiency, hyperthyroidism, and type 1 diabetes mellitus.